A third coronavirus vaccine has been approved for use in the UK, but doses will not be available until the spring.
The jab from US biotech firm Moderna has been given the green light by the Medicines and Healthcare products Regulatory Agency (MHRA), joining the vaccines from Pfizer/BioNTech and Oxford/AstraZeneca.
But unlike the previous jabs, the Moderna vaccine will not be available for use straight away, with the first doses not expected to arrive until the spring.
The Government also purchased an additional 10 million doses of the vaccine on top of its previous order of seven million, taking the total to 17 million.
Supplies will begin to be delivered to the UK from spring once Moderna expands its production capability, the Department of Health and Social Care said.
The MHRA accepted the recommendation of the Commission on Human Medicines and authorised the Moderna vaccine following months of rigorous clinical trials and extensive analysis of the vaccine’s safety, quality and effectiveness.
The jab is 94% effective in preventing disease, including in the elderly.
Prime Minister Boris Johnson tweeted that the approval was “excellent news”, adding: “Our national vaccine effort is accelerating to vaccinate priority groups with our existing two vaccines, and the Moderna doses will add to that when they become available in spring.”
Excellent news the @MHRAgovuk has approved the use of the @moderna_tx vaccine.
Our national vaccine effort is accelerating to vaccinate priority groups with our existing two vaccines, and the Moderna doses will add to that when they become available in spring. https://t.co/yt43dxGuGS
— Boris Johnson (@BorisJohnson) January 8, 2021
Health Secretary Matt Hancock said: “This is fantastic news and another weapon in our arsenal to tame this awful disease.
“Through our vaccine delivery plan, we have already vaccinated nearly 1.5 million people across the UK.
“The Moderna vaccine will boost our vaccination programme even further once doses become available from the spring.
“While we immunise those most at risk from Covid, I urge everyone to continue following the rules to keep cases low to protect our loved ones.”
The authorisation comes just days after the end of the Brexit transition period, and two days after the European Medicines Agency (EMA) recommended granting a conditional marketing authorisation for the jab for adults.
As part of the transition period, until the end of December 2020, Covid-19 vaccine candidates authorised via the EMA would have automatically been valid in the UK.
The Department of Health and Social Care said the Moderna vaccine will be available for free and the Government is working with the devolved administrations to ensure it is deployed fairly across the UK.
Like the other two vaccines, the Moderna vaccine will be deployed through hospital hubs for NHS and care staff and older patients to get vaccinated, through local community services with local teams and GPs, and through vaccination centres across the country.
Deputy chief medical officer for England Professor Jonathan Van-Tam said: “The highly effective Moderna vaccine is another impressive success for science and is another testament to the hard work of researchers and selfless clinical trial volunteers.
“This vaccine will save lives once doses become available, but it is crucial we all continue to follow the rules to protect each other until enough people have been protected.”
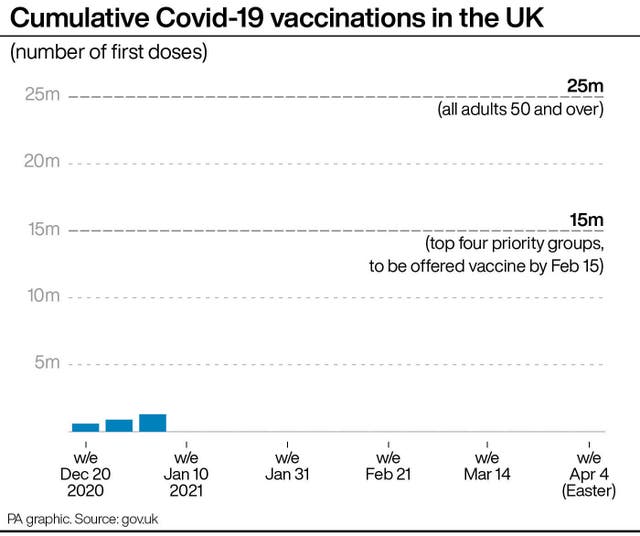
Nearly 1.5 million people in the UK have already been vaccinated with the Pfizer/BioNTech and Oxford University/AstraZeneca vaccines.
The Joint Committee on Vaccination and Immunisation (JCVI) will submit updated advice on which groups to prioritise for vaccination before doses become available.
Stephane Bancel, chief executive officer of Moderna, said: “We appreciate the confidence shown by the UK MHRA in Covid-19 vaccine Moderna with this decision – which marks an important step forward in the global fight against Covid-19.
“I want to thank the MHRA and the Commission on Human Medicines’ reviewers for their tireless efforts.
“The authorisation of a product developed by Moderna is a significant milestone on the company’s 10-year journey, and I would like to thank all our colleagues that have helped us get to this point.”
Vaccines are already being rolled out to those considered to be most vulnerable, according to a priority list guided by the JCVI.
The nine categories in phase one of the vaccination programme cover some 30 million people in the UK.
Mr Johnson has said the aim is to vaccinate 15 million people in the UK by mid-February, and officials hope to have inoculated all those in phase one by the spring.
The UK has now ordered 367 million doses of vaccines to protect against Covid-19.
This includes the Moderna doses – enough to vaccinate 8.5 million people – which are expected to be released in phases.
Like the Pfizer/BioNTech, the Moderna jab is an RNA jab which injects part of the virus’s genetic code in order to provoke an immune response.
It can be stored at temperatures of around minus 20C, compared to Pfizer/BioNTech’s minus 70C.
Jonathan Ball, professor of molecular virology, University of Nottingham, said: “It’s great news that another vaccine has been approved to help in the fight against the coronavirus pandemic.
“It is unclear when it will become available for distribution, but like the other mRNA vaccine, which is made by Pfizer, the reported effectiveness of the Moderna vaccine is very impressive.
“In an ideal world we would be able to prioritise the use of the mRNA vaccines to protect those most vulnerable from severe Covid disease, and the fact that the Moderna vaccine can be stored at minus 20 degrees, rather than minus 70 for the Pfizer vaccine, will help overcome some of the logistical challenges faced by the mRNA vaccines.
“We desperately need an effective vaccination programme so the more options available the better.”




Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules here